E-mail: sales001@bosschemical.com JINAN BOSS CHEM CO.,LTD
Telephone: +86-15628782329
| Availability: | |
|---|---|
| PDF Export | |





7447-41-8
bosschem
7447-41-8
Lithium chloride
CAS NO 7447-41-8
Alias:;LITHIUMCHLORIDE,CRYSTAL,REAGENT,ACS;LITHIUMCHLORIDE,POWDER,REAGENT,ACS
Molecular formula:LiCl
Molecular weight:42.39
Structural formula:
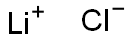
1.Performance and use
Lithium chloride has a wide range of uses, electrolytic production of lithium metal is the largest consumption of lithium chloride in the field, the current production of lithium metal is the only industrial method of lithium chloride fused salt electrolysis method, which was proposed by Guntz in 1893. Lithium metal and its alloys and compounds have a wide range of applications in the atomic energy industry, metallurgical industry, batteries, glass, ceramics, chemical industry, aerospace industry and many other fields.
1: Mainly used as raw materials for molten salt electrolysis to produce metallic lithium;
2: Used as air conditioning dehumidifier, insecticide, synthetic fiber, lithium battery, solar cell, bleach, metal alloy soldering agent or flux;
3: In the field of new materials, it serves as a catalyst for polymer materials such as polyphenylene sulfide and the production of chitin;
2.Technical indicators
| Product name | Lithium chloride |
| Appearance | White to gray |
| Melting point | 605°C(lit.) |
| Boiling point | 1383°C/1atm(lit.) |
| Density | 2.06 |
| Vapor pressure | 1.33hPa(547°C) |
| Refractive index | n20/D1.381 |
| Storage conditions | 2-8°C |
| Acidity coefficient (pKa) | 2.256[at20℃] |
| Solubility | H2O:soluble |
3. Packaging and storage
Packaging specification: 25kg/bag.
During transportation, it is necessary to protect against sunlight, rain, and high temperatures.
The product should be stored in a cool, dark, ventilated place, away from sources of fire.
4. Safety protection
Pay attention to labor protection when handling it and avoid contact with skin, eyes, etc.
After contact, rinse immediately with plenty of water
Related keywords:
Lithium chloride
CHLORIDE
CAS NO 7447-41-8
Industrial Grade
Lithium chloride
CAS NO 7447-41-8
Alias:;LITHIUMCHLORIDE,CRYSTAL,REAGENT,ACS;LITHIUMCHLORIDE,POWDER,REAGENT,ACS
Molecular formula:LiCl
Molecular weight:42.39
Structural formula:
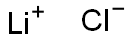
1.Performance and use
Lithium chloride has a wide range of uses, electrolytic production of lithium metal is the largest consumption of lithium chloride in the field, the current production of lithium metal is the only industrial method of lithium chloride fused salt electrolysis method, which was proposed by Guntz in 1893. Lithium metal and its alloys and compounds have a wide range of applications in the atomic energy industry, metallurgical industry, batteries, glass, ceramics, chemical industry, aerospace industry and many other fields.
1: Mainly used as raw materials for molten salt electrolysis to produce metallic lithium;
2: Used as air conditioning dehumidifier, insecticide, synthetic fiber, lithium battery, solar cell, bleach, metal alloy soldering agent or flux;
3: In the field of new materials, it serves as a catalyst for polymer materials such as polyphenylene sulfide and the production of chitin;
2.Technical indicators
| Product name | Lithium chloride |
| Appearance | White to gray |
| Melting point | 605°C(lit.) |
| Boiling point | 1383°C/1atm(lit.) |
| Density | 2.06 |
| Vapor pressure | 1.33hPa(547°C) |
| Refractive index | n20/D1.381 |
| Storage conditions | 2-8°C |
| Acidity coefficient (pKa) | 2.256[at20℃] |
| Solubility | H2O:soluble |
3. Packaging and storage
Packaging specification: 25kg/bag.
During transportation, it is necessary to protect against sunlight, rain, and high temperatures.
The product should be stored in a cool, dark, ventilated place, away from sources of fire.
4. Safety protection
Pay attention to labor protection when handling it and avoid contact with skin, eyes, etc.
After contact, rinse immediately with plenty of water
Related keywords:
Lithium chloride
CHLORIDE
CAS NO 7447-41-8
Industrial Grade
