E-mail: sales001@bosschemical.com JINAN BOSS CHEM CO.,LTD
Telephone: +86-15628782329
| Availability: | |
|---|---|
| PDF Export | |





12054-48-7
bosschem
12054-48-7
NICKEL HYDROXIDE (Ni(OH)2 )
CAS 12054-48-7
Alais:;NICKEL(+2)HYDROXIDE;NICKEL HYDROXIDE;Ni(OH)2;Niekelous hydroxide
Molecular formula: 12054-48-7
Molecular weight: H2NiO2
Structural formula:
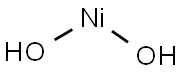
1. Performance and use
The chemical formula for nickel hydroxide is Ni (OH) 2 and NiO · xH2O. Green hexagonal crystal. Slightly soluble in water, easily soluble in acid and ammonia water, insoluble in liquid ammonia. When slowly dehydrated to 230 ℃ during heating, most of it becomes nickel (II) oxide, and complete dehydration requires red heat. It cannot be oxidized in air or hydrogen peroxide, but it is prone to form nickel (III) hydroxide in ozone. It can be oxidized by chlorine and bromine under alkaline conditions, but cannot be oxidized by iodine.
1. Nickel salt raw materials, alkaline batteries, electroplating, catalysts.
2. Used for alkaline battery production, nickel plating, etc;
3. Used for producing nickel salts, alkaline batteries, nickel plating, etc
2. Technical indicators
| items | standards |
| Ni+Co+Zn% | ≥60 |
| Co% | 0.6—2.0 |
| Zn%(Cd%) | 3.0±0.5 |
| Fe% | ≤0.005 |
| Ca% | ≤0.05 |
| Mg% | ≤0.05 |
| Cu% | ≤0.005 |
| SO42-% | ≤0.30 |
| NO3-% | ≤0.01 |
| CI-% | ≤0.02 |
| apparent density g/cm3 | ≥1.60 |
| tap density g/cm3 | ≥2.10 |
3. Packaging and storage
Package : 25kg/drum
Storage :In dry and cool places, avoid direct sunlight. The storage temperature should be kept at room temperature and sealed for storage
4. Related keywords
NICKEL HYDROXIDE (Ni(OH)2 )
CAS 12054-48-7
electroplate
Ni(OH)2
nickel hydroxide price
NICKEL HYDROXIDE (Ni(OH)2 )
CAS 12054-48-7
Alais:;NICKEL(+2)HYDROXIDE;NICKEL HYDROXIDE;Ni(OH)2;Niekelous hydroxide
Molecular formula: 12054-48-7
Molecular weight: H2NiO2
Structural formula:

1. Performance and use
The chemical formula for nickel hydroxide is Ni (OH) 2 and NiO · xH2O. Green hexagonal crystal. Slightly soluble in water, easily soluble in acid and ammonia water, insoluble in liquid ammonia. When slowly dehydrated to 230 ℃ during heating, most of it becomes nickel (II) oxide, and complete dehydration requires red heat. It cannot be oxidized in air or hydrogen peroxide, but it is prone to form nickel (III) hydroxide in ozone. It can be oxidized by chlorine and bromine under alkaline conditions, but cannot be oxidized by iodine.
1. Nickel salt raw materials, alkaline batteries, electroplating, catalysts.
2. Used for alkaline battery production, nickel plating, etc;
3. Used for producing nickel salts, alkaline batteries, nickel plating, etc
2. Technical indicators
| items | standards |
| Ni+Co+Zn% | ≥60 |
| Co% | 0.6—2.0 |
| Zn%(Cd%) | 3.0±0.5 |
| Fe% | ≤0.005 |
| Ca% | ≤0.05 |
| Mg% | ≤0.05 |
| Cu% | ≤0.005 |
| SO42-% | ≤0.30 |
| NO3-% | ≤0.01 |
| CI-% | ≤0.02 |
| apparent density g/cm3 | ≥1.60 |
| tap density g/cm3 | ≥2.10 |
3. Packaging and storage
Package : 25kg/drum
Storage :In dry and cool places, avoid direct sunlight. The storage temperature should be kept at room temperature and sealed for storage
4. Related keywords
NICKEL HYDROXIDE (Ni(OH)2 )
CAS 12054-48-7
electroplate
Ni(OH)2
nickel hydroxide price
